Pfizer seeks US authorization of Covid vaccine for children under five

- 2-02-2022, 09:47
INA- sources
Children under five, the last group of Americans still ineligible for vaccines against Covid-19, may soon receive emergency authorization for the shots, but getting all children vaccinated remains a serious challenge in the US.
Pfizer and its German pharmaceutical partner BioNTech announced on Tuesday that they were requesting emergency-use authorization of their vaccine for children aged six months to four years.
The application was submitted at the request of the US Food and Drug Administration, the companies said in a statement – an unusual move by the regulator.
The FDA’s independent advisers will meet on 15 February to discuss the application and the shots, containing just one-tenth of the dose given to adults, could be available to this population of 19 million in the US by the end of the month.
The agency’s decision could come within weeks, but that isn’t the only hurdle. The Centers for Disease Control and Prevention (CDC) also has to sign off, and many parents then have to be persuaded to get their young children vaccinated.
Haley Bryson post-Covid. For about two months, Haley had experienced some combination of headache, fatigue, stomachache, sore throat, earache or breathlessness.
The Biden administration has been trying to speed the authorization of Covid-19 shots for children, contending vaccinations are critical for opening schools and daycare centers and keeping them open, and for freeing parents from childcare duties so they can go back to work.
Many parents have been pushing for an expansion of shots to toddlers and preschoolers.
While children are much less likely to be hospitalized and die from Covid than adults, children’s hospitals have seen record-high admissions during the surge of the Omicron variant, especially in children who are not yet vaccinated.
Infants under a year old are most vulnerable to severe illness in the under-five age group.
The press release from the companies on Tuesday did not release any new data or updates to its study design.
In mid-December, Pfizer/BioNTech announced that two shots of a low dose created strong antibody responses in children between the ages of six months and two years.
But the doses produced disappointing results in children between the ages of two and four, prompting the addition of a third dose for all children under five in the trial. Data on the third shot is not expected until March at the earliest.
Regulators could now authorize the two-dose regimen for younger children, who saw effective responses to two shots. They might also allow slightly older children to receive the same doses in preparation for the third shot, should the data show an additional shot helps create a protective immune response.
“If two doses are authorized, parents will have the opportunity to begin a Covid-19 vaccination series for their children while awaiting potential authorization of a third dose,” Albert Bourla, chairman and CEO of Pfizer, said in the statement.
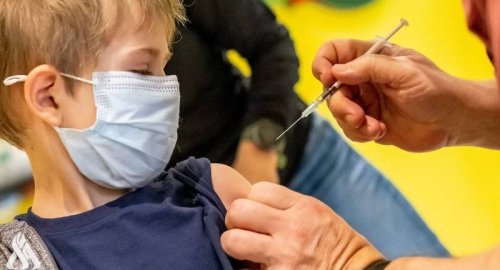
Shots for children aged five to 11 were authorized in October 2021. But only 22% of US children in that age group are fully vaccinated, and only three in 10 children who are eligible have received their first shot, according to the CDC.
“What we’re seeing right now is still a lot of hospitalizations and unfortunately some deaths in this age group,” said Dr Sean O’Leary of the University of Colorado, who is on the American Academy of Pediatrics’ infectious disease committee.
If the FDA clears vaccinations for these youngsters, he added, “that’s going to be really important because all of those hospitalizations and deaths essentially are preventable”.
A new report from the Kaiser Family Foundation found that 31% of parents would vaccinate their children under five right away – similar to what parents of over-fives said.
Two-thirds of parents worry about the Covid vaccines affecting their kids’ future fertility, according to an October 2021 survey.
In response, a new campaign aims to talk about fertility fears.
In fact, having Covid itself can temporarily affect men’s reproductive health; it’s unclear whether it has any short- or long-term effect on kids.
La Liga continues to pressure Barcelona
- Sport
- 09:47
Zionist airstrikes target the Damascus countryside
- International
- 09:07
Foreign Minister Invites Dutch Counterpart to Visit Iraq
- politics
- 06:38
Iraq Condemns Zionist Airstrikes on Syria
- politics
- 06:36
Al-Sistani: Tomorrow, the 29th of Ramadan
- Local
- 25/03/29
Al-Amiri warns of any war between Iran and the US
- politics
- 25/04/01