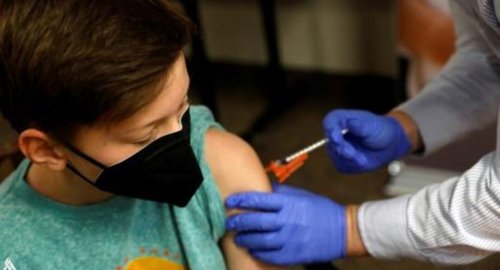
Switzerland approves Covid-19 vaccination for children aged between 5 and 11

- 11-12-2021, 08:42
INA-follow-up
The Swiss medicines agency Swissmedic on Friday approved the vaccination of children aged between five and 11 with Pfizer-Biontech's Comirnaty vaccine.
"Clinical trial results show that the vaccine is safe and effective in this age group," it said in a statement.
The Comirnaty vaccine is administered in two doses of ten microgrammes three weeks apart.
An ongoing clinical trial of more than 1,500 people "shows that the Covid-19 vaccine offers almost complete protection against serious illness caused by the SARS-CoV-2 virus in 5 to 11-year-olds", it said.
"Side effects tended to occur less frequently than in adolescents or adults. They included pain at the injection site and tiredness, or less frequently headache, aching limbs or fever," the agency added.
The vaccinations were until now limited to children aged 12 or older.
Switzerland is currently experiencing a strong fifth wave of the virus.
Only the Comirnaty and Moderna vaccine are authorized in Switzerland.
The country joins Portugal, Italy, Greece and Spain in Europe in giving the green light to the vaccination of children in this age group.
In France, vaccination has only been approved for young children at risk of developing serious illness but the government has said it is considering extending it to all children on a voluntary basis.
Trump: I will stop the chaos in the Middle East and the war in Ukraine
- International
- 10:07
US Central Command: We killed ISIS terrorist leader Abu Yusuf in Syria
- International
- 24/12/20
Liverpool compete with Real Madrid to sign Olympique Lyonnais star
- Security
- 24/12/19
ISC, ADX discuss Strengthening Economic Ties
- Economy
- 24/12/16
Iraq assumes presidency of Arab Investment Company’s Executive Board
- Economy
- 24/12/17












